Table of Contents
Why Are The SHO Gases in Nebulae Distributed Like That?
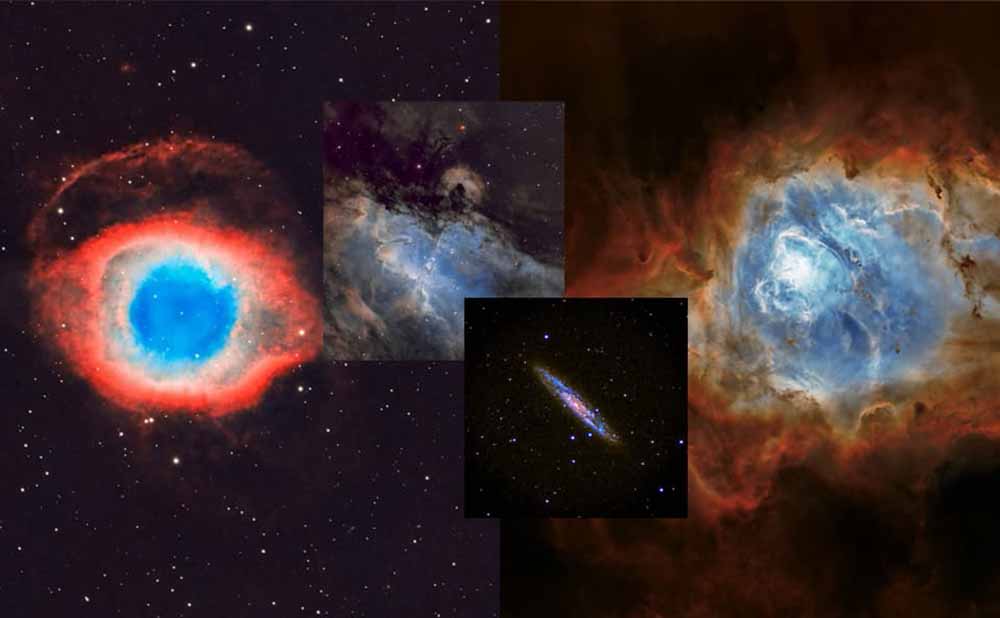
After a year of narrow-band imaging and sulfur-hydrogen-oxygen imaging, some astronomers noticed that the gas in the nebula is usually different from each other.
He also noticed that there is a repeated pattern in most nebulae. They usually see a blue cloud of oxygen in the middle, surrounded by a layer of sulfur and hydrogen outside.
What's The Physics Behind This?
Some astronomers suspect that it depends on the energy required to ionize the elements. If there is no ionization, the nebula will not glow, so it will not be visible. This means that OIII needs the maximum energy, even consistent with things like the California Nebula, where blue or OIII is the closest to the star that excites the nebula (the star is Menkib, which appears outside the nebula itself) Therefore, in this case, OIII almost seems to be in the "outside" of the nebula, and the main part is located in Ha and SII farther away from Menkib).
Therefore, this is not necessarily the distribution of elements in the nebula, it may depend on the distance from the excited star. Therefore, OIII requires higher energy, while Ha and SII require lower energy. Then, even if there is hydrogen or sulfur near the star, they will not be ionized at the required wavelength, because the energy is too high to make them fluoresce (the energy state is quantified).
This Guess May be Correct.
Atoms of different elements mix well in the whole jet, but the energy and quantity of ionized photons from the central star will vary with the distance from the star, the shielding of gas and dust, and other factors.
The O III line is produced by two ionized oxygen atoms. The removal of the first electron from the neutral oxygen atom requires about 13.6 electron volts (eV), while the removal of the second electron requires an additional 35.1 eV. Photons with such high energy are not common.
The S II line is produced by sulfur atoms that are ionized only once. It takes 10.4 eV to remove an electron from the neutral sulfur atom.
When the electron in the neutral hydrogen atom transitions from the n=3 level to the n=2 level, H will be formed- α。 There is no need to ionize the atom (this requires 13.6 eV), although this is the final emission of H- α A common mechanism of photons.
In short, compared with the creation of S II and H-alpha lines, the conditions for creating O III lines are much more extreme - higher energy photons are required. The scattering and absorption speed of high-energy short-wave ultraviolet photons that can produce OIII in peristellar matter is faster than that of low-energy photons, so the region that produces OIII will be closer to the photon source.
If you have more ideas, welcome to comment.

There are no customer reviews yet . Leave a Reply !